
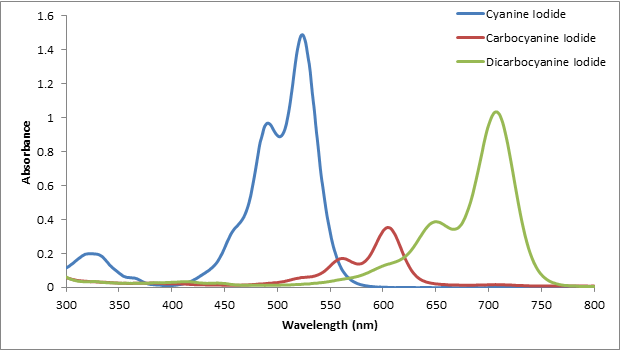
Figure 1 Direct (allowed) band gap Tauc plot (solid black) and absorption. Then subtracting the zero-point energy from $D_e$ to get $D_0$ and converting this value into kj/mol just to get an incorrect answer. of the UV-vis absorption spectrum can distinguish between these transitions. Then with $\omega_e$ and $\omega_ex_e$ I used the following formula to find the dissociation energy, $D_e$, I feel as though my error is in this part of the problem as the question asks for a transition from v = 0 but as far as I can see in the diagram there is no peak for the v = 0 transition.įollowing this, I converted the wavelengths for each transition into wavenumbers and then subtracted the wavenumber corresponding to each transition: Enter a wavelength between 200 nm and 700 nm and then click on start to begin the simulation. My first approach in solving this problem was selecting two transitions (v" = 2 to v' = 7 and v" = 3 to v' = 8). Create a graph of absorbance vs wavelength. The question calls for an estimation of the energy (in kj/mol) required to dissociate ClO in its excited state when it is excited from the v = 0 of the ground state. I have been having trouble solving the following problem.


 0 kommentar(er)
0 kommentar(er)
